how to draw molecular orbital diagram of o2
Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. I wouldnt violate the au.
What Is The Molecular Orbital Diagram For Oxygen Quora
Note that the textbook and my hand-drawn answer start at the 2sigma MO not the 1sigma MOthats just shorthand for ease of drawing since MOs below 2sigma do not vary in energy level or number of electrons in.
. The last two electrons in p 2px. Solution We draw a molecular orbital energy diagram similar to that shown in. Iii compounds formed when an element of group 2 combines with an element of group 16.
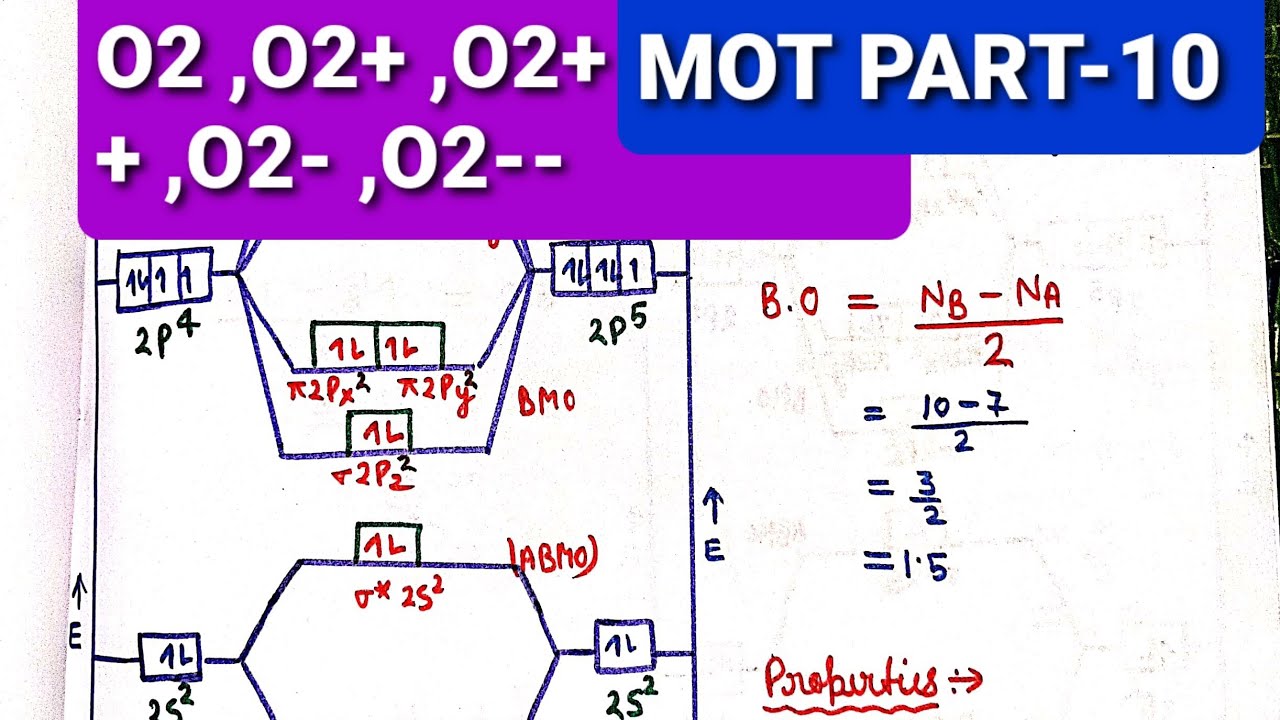
Bond order is defined as half of the difference between the number of electrons present in the bonding and antibonding orbitals. That means with 10 AOs in you get 10 MOs. Number of electrons in bonding orbitals.
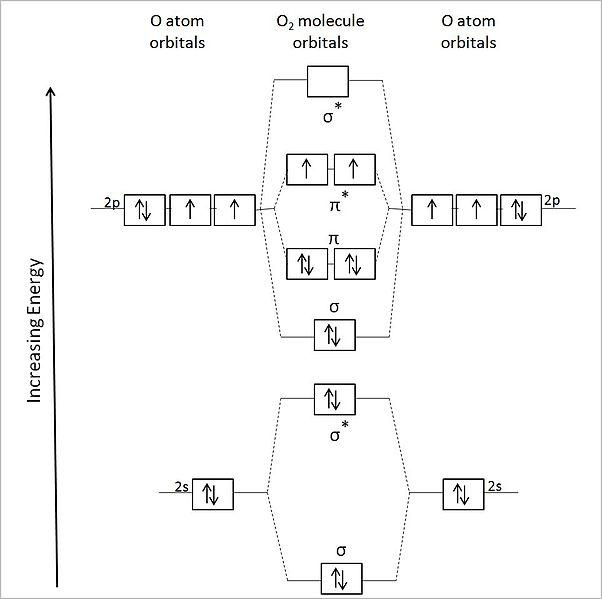
Thus oxygen molecule has two bonds. In order to draw oxygens molecular orbital diagram you need to start by taking a look at what atomic orbitals you have for an oxygen atom o. The molecular orbital energy level diagram of oxygen molecule is given as follows.
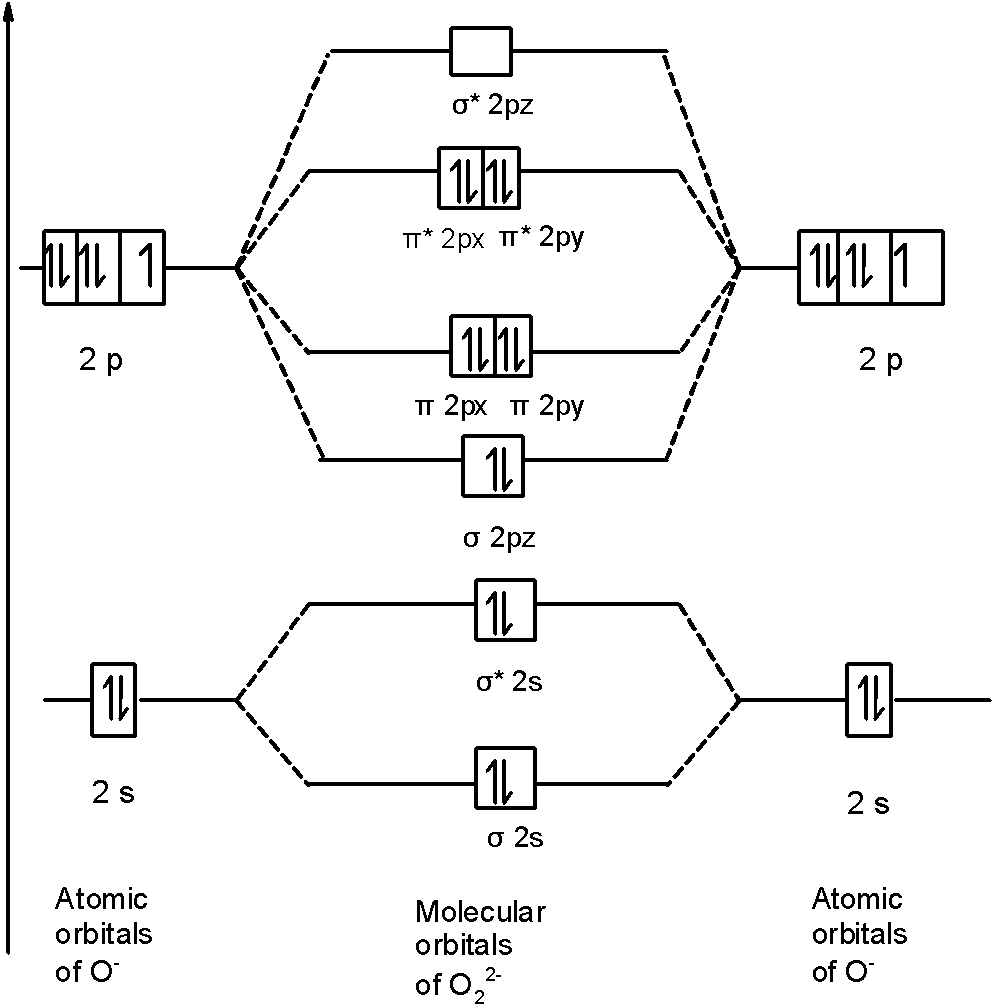
Because of the difference in their atomic orbital energies the 1s orbital of hydrogen and the 3s orbital of sulfur interact only weakly. From that diagram you can then easily fill out what the O2- and O2 MO diagrams should beand that is in the second photo I included. From this diagram calculate the bond order for O 2.
Next well see that symmetry will help us treat larger. The purpose of MO theory is to fill in the gap for some. Draw similar diagrams for other orbitals in the print outThe first photo is straight from a edition Pearson general chemistry textbook and it shows you what the molecular orbital MO diagram for O2 is.
Sorry the Sigma-1-s orbital is a little off-screen. Q1 a Give the molecular orbital diagram of O2. Based on the grouped of elements state the formula for the following.
Molecular Orbital MO Theory is the final theory pertaining to the bonding between molecules. So your first molecular orbital should have 0 nodes and then increase with increase by one with each increasing energy level so the more energy levels you have you would just increase the number of nodes by one each. This is shown in the diagram by a slight stabilization of the lowest.
Molecular Orbital Diagram for Oxygen Gas O2Fill from the bottom up with 12 electrons totalBonding Order is 2 and it is Paramagneticsigma2s2sigma2s. Figure Molecular Orbital Energy-Level Diagram for pi Each oxygen atom in ozone has 6 valence electrons so O 3 has a total of eV. Ie one is bond and one p bond.
Each oxygen atom contributes six electrons so the diagram appears as shown in. T of group 13. Orbitals will remain unpaired.
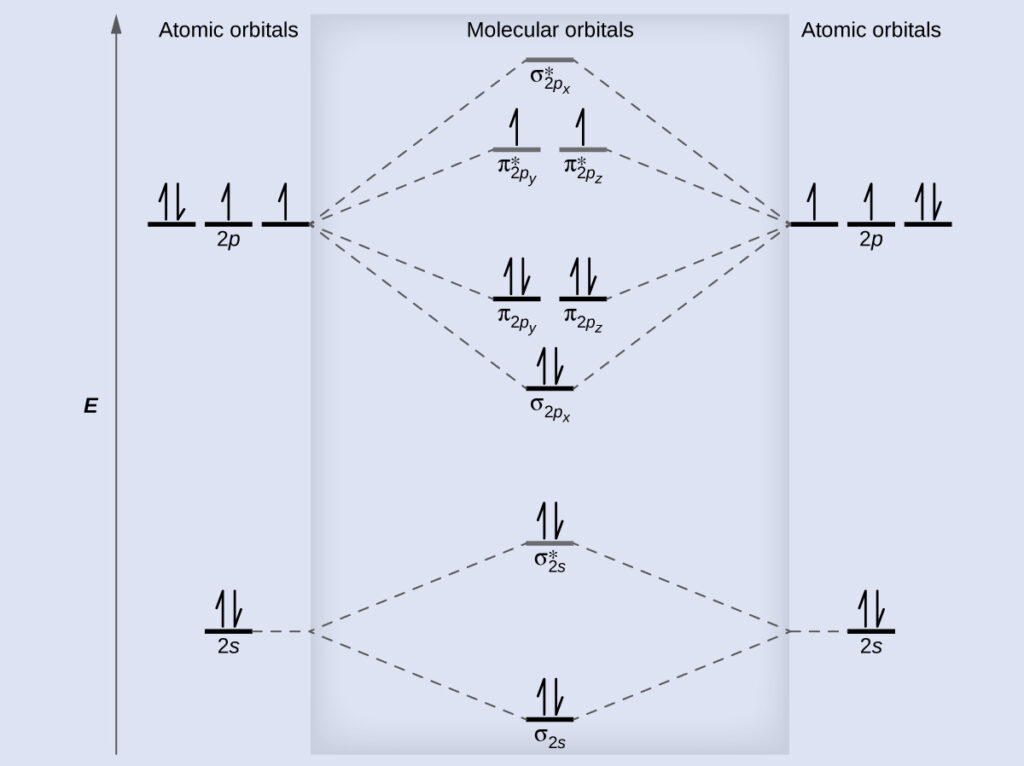
Each oxygen atom combines its 2s 2p z and 2p ddiagram orbitals to make three 2sp 2 hybrid orbitals. For a homonuclear diatomic molecule like O2 this is simple. In contrast to VSEPR and valence bond theory which describe bonding in terms of atomic orbitals molecular orbital theory visualizes bonding in relation to molecular orbitals which are orbitals that surround the entire molecule.
How to draw the molecular orbital diagram of O2. The applications of the mo theory extend beyond the limitations of the valence shell electron pair repulsion vsepr model and the valence bond theory. Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals.
ANSWER ACCORDING TO PERIODIC CLASSIFICATION OF ELEMENTS CLASS X CBSE. Draw the molecular orbital diagram of o2 and calculate the bond. It is analogous to the atomic orbital energy diagram which goes 1s 2s 2p 3s.
- N 2 molecules are diamagnetic with no unpaired electrons. How to build molecular orbitals. The O2- MO diagram will have one more electron compared to O2 and the O2 MO diagram will have one fewer electron compared to O2.
Then for the molecular orbital diagram we examine how these atomic orbitals interact with each other in a linear combination of atomic orbitals LCAO. Draw the molecular orbital diagram for the oxygen molecule O 2. These interactions generate what are called molecular orbitals and they will conserve the number of orbitals.
Bond order 1 2 Number of electrons in BMO Number of electrons in ABMO Bond order 1 2 8 2 Bond order 1 2 6 Bond order 3. A molecular orbital diagramor MO diagramis a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals LCAO molecular orbital method in particular. Draw the MO for O 2.
Summary MO Theory LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. BO 12 bonding e- - antibonding e- 122222 - 21 colorblue25 And this should make sense because NO is isoelectronic with CO which has a bond order of 3. Therefore oxygen molecule has paramagnetic character due to the presence of two unpaired electrons.
So you must always be flipping it back and forth 4 the number of nodes in your molecular orbitals must always begin at 0. I oxides of 1st group elements ii allis of the elemen. For the O2 O2- 2 and O22 assign the HOMO LUMO the bond order magnetic properties and compare between the bond energy bond length and ionization energy.
Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. Fill those with oxygens 8 electrons 2 core 6 valence. Rest assured its filled.
Heres how this goes of course the ns are compatible with the ns. So the 2s interacts with the 2s and the 2p interacts with the 2p etc. Just choose the orbitals that are alike in look and energy.
How does this diagram account for the paramagnetism of O 2. Bond order 2nb na 284 2. The other is for AFTER nitrogen start.
Number of electrons in antibonding orbitals. So the formula to find bond order is. B Draw πp πp and σ p.
O2 kk σ2s 2 σ2s 2 π2p x 2 π2p y. Molecular Orbital Diagrams. Now we have two of the same atomic orbital diagrams laid out.
What Is The Molecular Orbital Diagram For Oxygen Quora

Draw Molecular Orbital Diagram For O2 Molecule Brainly In

Draw The Valence Shell Molecular Orbital Diagram Of Oxygen Molecule And Predict Its Magnetic Nature

Explain The Formation Of O2 Molecule By Molecular Orbital Theory M O T Brainly In

Construct Molecular Orbital Diagram And Determine Unpaired Electrons In O2 O2 Bn No Study Com

Draw The Molecular Orbital Diagram Of Dioxygen And Calculate Bond Order

8 Drawing Molecular Orbital Diagrams Flux Science
Why Is The Molecular Orbital Diagram For O Different From N Quora

Draw The Molecular Orbital Energy Diagram For Oxygen Molecule O2 And Show That I It Has A Double Bond Ii It Has Paramagnetic Character From Chemistry Chemical Bonding And Molecular Structure Class 11 Cbse
Draw The Molecular Orbital Diagram Of Dioxygen And Class 11 Chemistry Cbse

Draw Molecular Orbital Diagram Of O2 Or N2 With Magnetic Behavior And Bond Order

Draw A Molecular Orbital Diagram Of N2 Or O2 With Magnetic Class 11 Chemistry Cbse

Draw The Molecular Orbital Diagram Of O2 And Calculate The Bond Order Is O2 Diamagnetic Or Paramagnetic Explain Your Answer Study Com

Explain The Formation Of O2 Molecule By Molecular Orbital Theory
Bond Order Ofo2 O2 O2 And O22 Is In Order A O2 Langle Class 11 Chemistry Cbse
Mo Diagram O2 O2 2 O2 O2 2 Preparation Of Gate Csir Net Uset Set Exam Youtube
Molecular Orbital Diagrams Bond Order And Number Of Unpaired Electrons Chem Textbook

Molecular Orbital Theory Homodiatomics Use The Molecular Orbital Model To Fully Describe The Bonding In O2 O2 O2 And O22 Determine Which Of The Following Statements Are True And Which Are